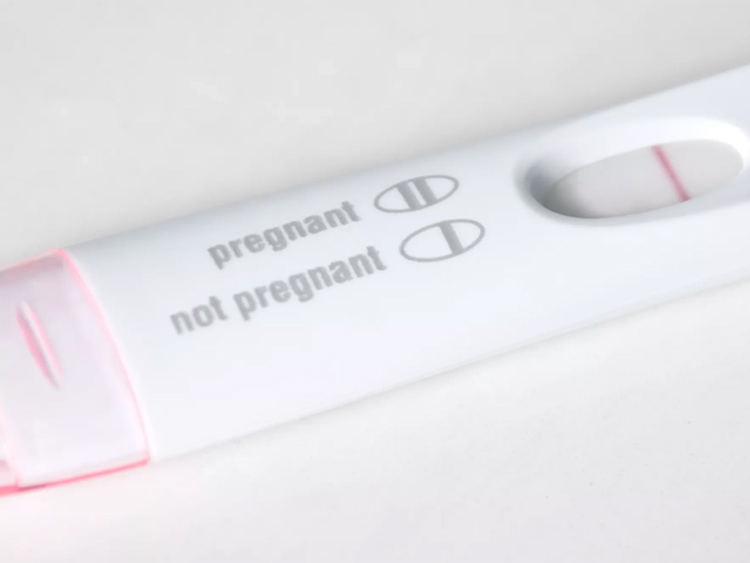
Velo hCG Pregnancy Rapid Test
Catalog Number: VE242001
Package Specification: 20 tests/kit
Principle
Human Chorionic Gonadotropin (hCG) and Pregnancy Detection
Human chorionic gonadotropin (hCG) is a glycoprotein hormone produced by the developing placenta shortly after fertilization. It can be detected in urine, serum, or plasma as early as 7 to 10 days after conception in normal pregnancy. hCG levels rise rapidly, often exceeding 100 mIU/mL by the first missed menstrual period, and peaking in the 100,000-200,000 mIU/mL range around 10-12 weeks into pregnancy. The appearance of hCG in bodily fluids shortly after conception, and its subsequent rapid increase during early gestational growth, make it a reliable marker for early pregnancy detection.
Velo Human Chorionic Gonadotropin (hCG) Rapid Test
The Velo Human Chorionic Gonadotropin (hCG) Rapid Test is a qualitative rapid test designed to detect hCG at a sensitivity of 25 mIU/mL in urine. This test employs a combination of monoclonal and polyclonal antibodies, including mouse monoclonal anti-hCG antibodies, to selectively detect elevated levels of hCG in urine, serum, or plasma samples. The assay is performed by adding a urine specimen to the specimen well of the test cassette. The specimen migrates along the membrane via capillary action to react with the colored conjugate present. Positive specimens react with specific colored antibody conjugates, producing a colored line at the test line region of the membrane. A lack of this colored line indicates a negative result. Additionally, to ensure the test’s procedural integrity, a colored line will always appear at the control line region if the test has been executed correctly.
Sample Collection and Preparation
Materials Provided
Each kit contains the following components:
- Test Devices: 20 individual test devices, each securely pouched for convenience and hygiene.
- Droppers: 20 droppers, each capable of dispensing 25 μl of specimen, facilitating precise and accurate testing.
- Package Insert: 1 comprehensive piece attached, offering detailed instructions and essential information for the correct usage and interpretation of the test.
Additional Materials Needed (but Not Included in the Kit)
- Timer or Stopwatch: Required for accurate timing during the testing process.
- Specimen Collection Containers: Necessary for collecting urine, serum, or plasma samples prior to testing.
- Disposable Gloves and/or Protective Clothing: Recommended to maintain hygiene and safety during specimen collection and testing procedures.
- Micropipette: Useful for precise measurement and transfer of specimen volumes, particularly if not provided in the kit.
Sample collection
Urine Assay
Collect a urine specimen in a clean, dry container. While a first-morning urine specimen is preferred due to its typically higher hCG concentration, specimens collected at any time of the day may be used. If the urine specimen shows visible precipitates, it should be centrifuged, filtered, or allowed to settle to obtain a clear specimen for testing.
Serum or Plasma Assay
Aseptically collect blood into a clean tube without anticoagulants (Serum) or with anticoagulants (Plasma). Separate the serum or plasma from the blood as soon as possible to prevent hemolysis. Whenever feasible, use clear, non-hemolyzed specimens.
Specimen Storage
Urine, serum, or plasma specimens can be stored at 2-8°C for up to 48 hours before testing. For longer storage periods, specimens may be frozen and stored below -20°C. Prior to testing, thaw and mix frozen specimens thoroughly.
Procedure
Before proceeding with the test, ensure that both the tests and specimens have reached room temperature (15-30ºC).
Allow the test device and specimens to reach room temperature before opening the pouch. Remove the test device from the sealed pouch and use it promptly.
Testing
Place the test device on a clean, level surface. Hold the dropper vertically and transfer 2 full drops of urine (approximately 50 μL) to the specimen well of the test device. Start the timer immediately after adding the specimen. Avoid trapping air bubbles in the specimen well. Refer to the illustration below for guidance.
Quality Control
The test includes an internal procedural control. A colored line appearing in the control line region (C) serves as an internal valid procedural control, confirming adequate membrane wicking. While control standards are not supplied with this kit, it is recommended as good laboratory practice to test positive and negative controls. This practice helps confirm the test procedure and verify proper test performance.
Results
Wait for the colored line(s) to appear. Read the results within 5 minutes of adding the specimen. Avoid interpreting the result after 10 minutes.

Interpretation of Results
(Please refer to the illustration above)
- Negative Result: If only the quality control line C is visible and the detection line remains colorless, it indicates that hCG has not been detected, resulting in a negative outcome.
- Positive Result: When both the quality control line C and the detection line T are present, it signifies the detection of hCG, resulting in a positive outcome.
- Invalid Result: In the absence of the quality control line C, regardless of the presence or absence of the detection line, the result is considered invalid. In such cases, the test should be repeated.
Specific Characteristics
Accuracy
A multi-center clinical evaluation was conducted comparing the results obtained using Velo Human Chorionic Gonadotropin (hCG) Rapid Test to another commercially available urine and serum or plasma hCG Rapid test. The urine study included 413 specimens, and both assays identified 296 negative and 117 positive results. The serum study included 200 specimens, and both assays identified 141 negative and 59 positive results. The plasma study included 200 specimens, and both assays identified 141 negative and 59 positive results. The results demonstrated a >99% overall accuracy of Velo Human Chorionic Gonadotropin (hCG) Rapid Test when compared to the other urine and serum or plasma hCG Rapid test.
| hCG Reference Method (Urine) | ||||
| Method | Other hCG Rapid Test | Total Results | ||
| Velo Human Chorionic Gonadotropin (hCG) Rapid Test | Results | Positive | Negative | |
| Positive | 117 | 0 | 117 | |
| Negative | 0 | 296 | 296 | |
| Total Results | 117 | 296 | 413 | |
| Sensitivity: 100% (96.9%~100%) | Specificity: 100% (98.8%~100%) | |||
| Accuracy: 100% (99.1%~100%) | 97.5% Confidence Intervals | |||
| hCG Reference Method (Serum) | ||||
| Method | Other hCG Rapid Test | Total Results | ||
| Velo Human Chorionic Gonadotropin (hCG) Rapid Test | Results | Positive | Negative | |
| Positive | 59 | 0 | 59 | |
| Negative | 0 | 141 | 141 | |
| Total Results | 59 | 296 | 200 | |
| Sensitivity: 100% (93.9%~100%) | Specificity: 100% (97.4%~100%) | |||
| Accuracy: 100% (98.2%~100%) | 97.5% Confidence Intervals | |||
| hCG Reference Method (Plasma) | ||||
| Method | Other hCG Rapid Test | Total Results | ||
| Velo Human Chorionic Gonadotropin (hCG) Rapid Test | Results | Positive | Negative | |
| Positive | 59 | 0 | 59 | |
| Negative | 0 | 141 | 141 | |
| Total Results | 59 | 296 | 200 | |
| Sensitivity: 100% (93.9%~100%) | Specificity: 100% (97.4%~100%) | |||
| Accuracy: 100% (98.2%~100%) | 97.5% Confidence Intervals | |||
Interfering substances
A study was conducted to determine the following potentially interfering substances, which were added to hCG negative and positive specimens, then tested with Velo Human Chorionic Gonadotropin (hCG)) Rapid Test, tested no interfered.
| Compounds | Conc.mg/dL | Compounds | Conc.mg/dL |
| Acetaminophen | 20 | Acetone | 1000 |
| Acetylsalicylic Acid | 20 | Acetoacetic Acid | 2,000 |
| Ampicillin | 20 | Ascorbic Acid | 20 |
| Atropine | 20 | Albumin | 2,000 |
| ß-Hydroxybutyrate salt | 2000 | Benzoylecgonine | 10 |
| Bilirubin | 20 | Brompheniramine | 20 |
| Caffeine | 20 | Cannabinol | 10 |
| Clomiphene | 100 | Cocaine | 10 |
| Codeine | 10 | Cholesterol | 500 |
| Creatine | 20 | Dextromethorphan | 20 |
| DMSO | 5% | EDTA | 80 |
| Ephedrine | 20 | Ethanol | 1% |
| Estriol | 2 | Estrone 3-Sulfate | 10 |
| Gentisic Acid | 20 | Glucose | 2,000 |
| Hemoglobin | 1,000 | Heroin | 1 |
| Ibuprofen | 20 | Methadone | 10 |
| Methamphetamine | 10 | Methanol | 10% |
| Morphine | 0.6 | Oxalic Acid | 40 |
| Phenothiazine | 20 | Phenylpropanolamine | 20 |
| Pregnanediol | 2 | Salicylic Acid | 20 |
| Tetracycline | 20 | Triglycerides | 1,200 |
| Theophylline | 20 | Urea | 2,000 |
| Uric Acid | 20 | —– | |
Cross-Reactivity
A study was conducted to determine the cross-reactivity of the test with compounds in either negative or positive specimen. The test has been standardized to the W.H.O. Fourth International Standard (75/589). The addition of LH (300 mIU/mL), FSH (1,000 mIU/mL), and TSH (1,000 µIU/mL) to negative (0 mIU/mL hCG) and positive (25 mIU/mL hCG) specimens showed no cross-reactivity with Velo Human Chorionic Gonadotropin (hCG) Rapid Test.
Limitation of the Procedure
- Velo Human Chorionic Gonadotropin (hCG) Rapid Test is a preliminary qualitative test, therefore, neither the quantitative value nor the rate of increase in hCG can be determined by this test.
- Very dilute urine specimens, as indicated by a low specific gravity, may not contain representative levels of hCG. If pregnancy is still suspected, a first morning urine specimen should be collected 48 hours later and tested.
- Very low levels of hCG (less than 50 mIU/mL) are present in urine specimen shortly after implantation. However, because a significant number of first trimester pregnancies terminate for natural reasons, a test result that is weakly positive should be confirmed by retesting with a first morning urine specimen collected 48 hours later.
- This test may produce false positive results. A number of conditions other than pregnancy, including trophoblastic disease and certain non-trophoblastic neoplasms including testicular tumors, prostate cancer, breast cancer, and lung cancer, cause elevated levels of hCG. Therefore, the presence of hCG in urine should not be used to diagnose pregnancy unless these conditions have been ruled out.
- This test may produce false negative results. False negative results may occur when the levels of hCG are below the sensitivity level of the test. When pregnancy is still suspected, a first morning urine specimen should be collected 48 hours later and tested. In case pregnancy is suspected and the test continues to produce negative results, see a physician for further diagnosis.
- As with any assay employing mouse antibodies, the possibility exists for interference by human anti-mouse antibodies (HAMA) in the specimen. Specimens from patients who have received preparations of monoclonal antibodies for diagnosis or therapy may contain HAMA. Such specimens may cause false positive or false negative results.
This test provides a presumptive diagnosis for pregnancy. A confirmed pregnancy diagnosis should only be made by a physician after all clinical and laboratory findings have been evaluated.
Storage and Safety
Storage
Store the Velo Human Chorionic Gonadotropin (hCG) Rapid Test kit between 2°C and 30°C. The shelf life of the kit is 36 months.
If stored refrigerated, ensure that the pouched device is brought to room temperature before opening.
Do not freeze the kit.
Warnings
- Read the Package Insert carefully: Ensure to thoroughly read the entire package insert before using the product. Failure to follow the instructions may lead to inaccurate results.
- For Diagnostic Use Only: The Velo Human Chorionic Gonadotropin (hCG) Rapid Test is intended for diagnostic use only. It is not intended for screening purposes.
- Perform Test at Room Temperature: Conduct the test at room temperature to ensure accurate results. Failure to do so may affect the accuracy of the results.
Precautions
- The Velo Human Chorionic Gonadotropin (hCG) Rapid Test is intended for professional use only.
- Adhere to the instructions provided in the package insert to ensure optimal test performance.
- Discard used test devices according to local regulations.
Handling Precautions
- Ensure not to use the kit if the safety seal on the kit box is missing, damaged, or broken.
- Avoid using the kit if the pouches have been perforated.
- Each device is for single use only.
- Refrain from using the kit after the expiration date marked on the kit box.
- Adequate lighting is required to read the test results accurately.
- Read the results promptly after the 5-minute incubation period following the addition of the specimen. Results should not be read after 10 minutes.